Dalton's Law of Partial Pressure
The partial pressure is the pressure that each gas would exert if it alone occupied the volume of the mixture at the same temperature. Therefore the directions of diffusion during gas exchange in the lungs and in body tissues are based on the differences in partial pressure of.

Dalton S Law Of Partial Pressureslaw Dalton Pressures Partial Dalton S Law Basic Physics Thermodynamics
In 1803 he revealed the concept of Daltons Law of Partial Pressures.
. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 15 atm on the walls of its container. P i the. If the partial pressure of hydrogen is 1 atm find the mole fraction of oxygen in the mixture.
V 2 n 2. P tot the total pressure. Triple Point - Triple points for common substances.
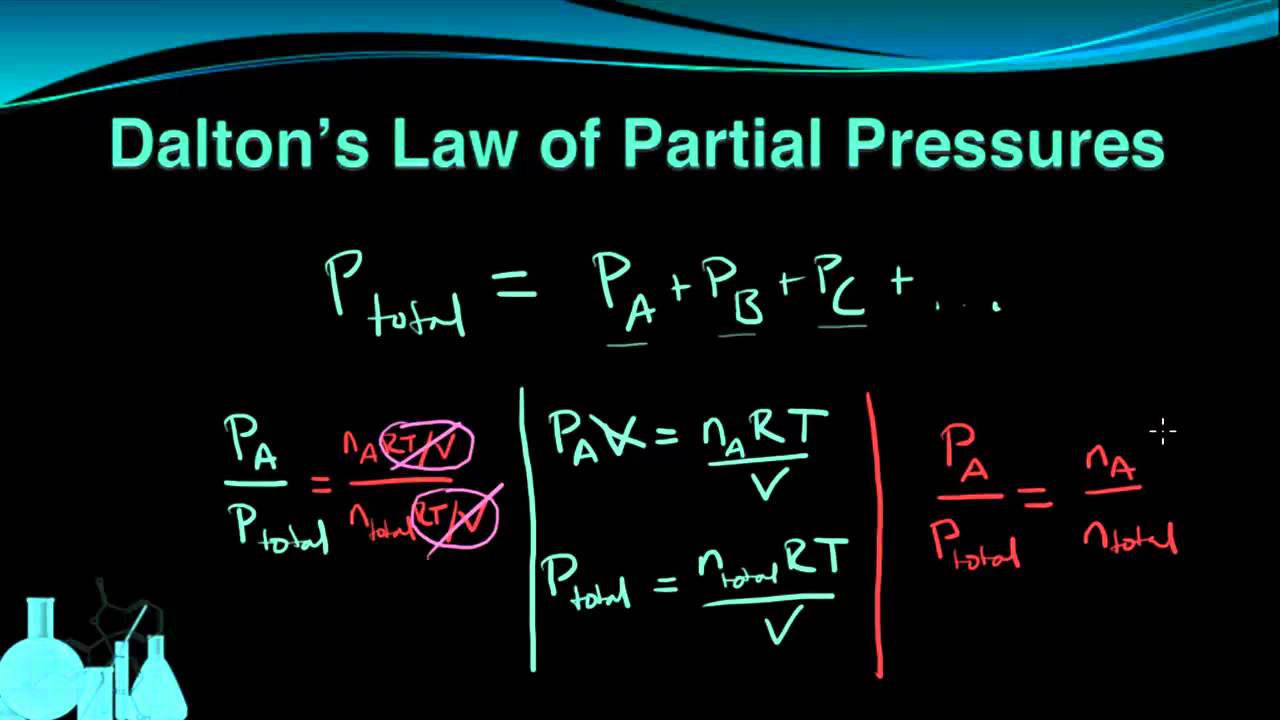
Daltons law of partial pressures states that the pressure of a mixture of gases simply is the sum of the partial pressures of the individual components. Partial Pressure- Partial Pressure is defined as a container filled with more than one gas each gas exerts pressure. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture Daltons Law.
Also in the 1800s he was the first scientist to explain the behavior of. Visit BYJUS for more content. P p 1 p 2 p 3.
Concentration and MolarityPreparing a Stock Solution. The limitations of Raoults Law are as follows. Where P is the mole fractions of the components.
Limitations of Raoults Law. To Learn expressions on Daltons law of partial pressure Examples Videos with FAQs. Total and Partial Pressure - Daltons Law of Partial Pressures - How to calculate total pressure and partial pressures for gas mixtures from Ideal Gas Law.
Although a schoolteacher a meteorologist and an expert on color blindness John Dalton is best known for his pioneering theory of atomism. Sum of all partial pressure is the total pressure of that gas mixture a physical law called the. Concentration and MolarityFinding Concentration of Ions in an Aqueous Solution.
The term partial pressure is used when we have a mixture of two or several gases in the same volume and it expresses the pressure that is caused by each of the induvidual gases in the mixture. Definition of partial pressure and using Daltons law of partial pressures. Understand Daltons Law of Partial Pressures.
The total pressure of the gas mixture is the sum of the partial pressure of the component gases. This new pressure is the partial pressure of each A and B and is given by Raoults law and depends on the concentration of each component in the liquid phase. The gases present in the container are chemically inert.
The Ideal Gas Law - The relationship between volume pressure temperature and quantity of a gas including definition of gas density. PA XA PB XB XAPA XBPB. This empirical relation was stated by the English chemist John Dalton in 1801.
He also developed methods to calculate atomic weights and structures and formulated the law of partial pressures. Solved Examples on Daltons Law of Partial Pressure Example 1. In a mixture of gases each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature.
Avogadros law states that equal volumes of all gases at the same temperature and pressure have the same number of molecules. Concentration and MolarityDetermine a Concentration From A Known Mass of Solute. P tot P i P 1 P 2 P 3.
Early Life dalton1-profilejpg John Dalton FRS engraved by William Henry Worthington after an 1814 painting by William. Daltons Law of Partial Pressures. Daltons law the statement that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual component gases.
If youre seeing this message it means were having trouble loading external resources on our website. Developed by chemist and physicist John Dalton who first advanced the concept of chemical elements being made up of atoms 9 X Research source Daltons Law states that the total pressure of a gas mixture is the sum of the pressures of each of the gases in the mixture. The pressure of any gas within the container is called its partial pressure.
The partial pressure of a gas is a measure of. Charles Gas Law.

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas

Dalton S Law Of Partial Pressure Ideal Gas Equation Gases And Kinetic Molecular Theory Chemistry Khan Ac Dalton S Law Anatomy And Physiology Physiology

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry

Dalton S Law Of Partial Pressures Explained Dalton S Law Medical Anatomy Respiratory Therapy

Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study
0 Response to "Dalton's Law of Partial Pressure"
Post a Comment